The brain is different compared to other organs in various aspects, including its regenerative capacities: Traditionally it was believed, that the hardware of the brain is “that hard” that once the brain is injured, brain areas and functions are lost forever. This old paradigm of the adult CNS as a stable and static structure has now been replaced by a much more dynamic view of the CNS. We know from clinical studies and animal models, that an injury to the brain induces plastic mechanisms on different levels including macro-anatomical functional map-shifts, dendritic and spine-turnover as well as genomic and proteomic modifications. Although these intrinsic repair mechanisms exist which allow forms of spontaneous recovery – e.g. a stroke patient who regains some control of a paretic hand without further treatment – the intrinsic repair mechanisms are often not sufficient to promote full recovery of impaired function. Furthermore, the neuropathological mechanisms how ischemic events induce cognitive decline as a long-term consequence are not understood at all, reflected by no specific treatment options available to prevent or attenuate memory deficits.
A prerequisite for developing novel therapeutic approaches, is to improve our understanding, how the brain itself deals with structural damage on different levels- from a network, to a cellular and subcellular level. Thus, the goal of our work is, to uncover the principles of neuronal repair and rewiring to identify new targets for restoring impaired neuronal activity and for promoting recovery of lost function after brain injury.
Specifically, we would like to understand, how some nerve cells are chosen to participate in repair processes while other nerve cells maintain their old function. We also examine how surviving nerve cells form new connections, how old connections are strengthened, which neuronal circuits reorganize, stabilize or disintegrate and how neuronal remodeling contributes to the functional outcome.
Elucidating causal connections between neuronal rewiring and functional outcome
We use different imaging methods in the behaving animal (performing grasping tasks, running on a running wheel or in a virtual reality environment) during learning and after the induction of different stroke models. We also apply opto- and chemogenetics, sophisticated behavioral assessments for sensorimotor and cognitive functions as well as deep learning algorithms to explore causal relationships between neuronal rewiring- from a cellular resolution till a network level- and the behavioral phenotype.
Studying neuronal microcircuits for sensorimotor dysfunction and recovery after stroke
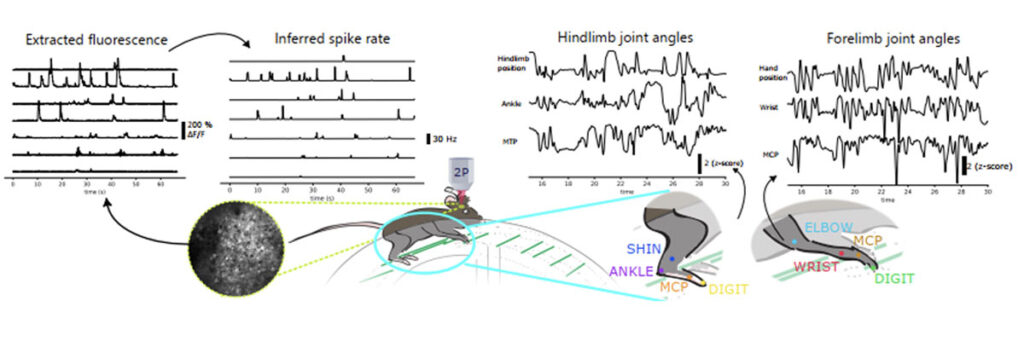
We study how individual surviving neurons in the cortex rewire and recode to take over lost- or impaired motor function after a photothrombotic stroke targeting the sensorimotor cortex. We use chronic 2-photon calcium imaging in active animals following the same region of interest in the cortex during learning of the motor task and after stroke. We hope to reveal (i) how the computation of individual neurons and neuronal ensembles adapt to injury and how this is related to motor recovery, (ii) how different layers of the motor cortex contribute to the rewiring processes, (iii) if mechanisms of spontaneous recovery can be further promoted by appropriate external treatment approaches (e.g. pharmacology or rehabilitation).
Video: 2photon imaging of hindlimb motor neurons
Studying the pathomechanisms of cognitive decline on a microcircuit level after stroke
Almost 20% of stroke patients develop forms of cognitive decline months but also years after the ischemic event. The underlying mechanisms are not understood. A critical area of memory formation but also memory degeneration is the hippocampus. Hippocampal neurons are in particular known to be highly vulnerable to ischemic injury. Using chronic 2-photon calcium imaging in the hippocampus during learning of a spatial memory task (mice learn to navigate in a virtual reality set-up) and after induction of micro-strokes we hope to elucidate (i) how micro-strokes impair the local neuronal circuitry in the hippocampus affecting memory, (ii) how surviving neurons and functional ensembles of neurons rewire to compensate for the damage and (iii) if neuronal rewiring can be further enhanced by the appropriate stimulation paradigm or neuro-cognitive rehabilitation schedule.
Video: 2photon imaging of CA1 neurons
